A Virology Primer: With Special Reference to Ozone
Revised October 2001by Gérard V. Sunnen, M.D.
First published September 22, 1997
BACK TO HOMEViruses are intracellular parasites. They require a living host cell in order to replicate and to infect new hosts. Viruses have been enormously successful in parasitizing most known forms of living organisms in both the animal and plant world.
- Nucleic acid core. At the core of viruses is genetic material which encodes the transcription of all viral components, mostly proteins (such as enzymes). This genetic material is either RNA or DNA, never both. The nucleic acid may be single or double stranded. Viral nucleic acid has been described as the software program for making copies of the virus.
- The nucleic acid coat or capsid. Surrounding the core is a protective coating made of protein, called the capsid. The capsid is rigid and determines the shape of the virus. It is made of repeating protein units called capsomeres.
The architecture of capsomere assembly is quite fascinating. A very common crystal-like configuration is the 20-sided isocahedral construction, with each capsomere forming an equilateral triangle.
Another main pattern is helical or rod shape. The nucleic material and its capsid curls unto itself like a snake, forming a spherical structure.
A few viruses have capsids that are neither icosahedral nor helical; these configurations are termed complex, and may be spiral, brick shaped, or may show other non-standard appearances.
The capsid and its nucleic acid core together form the nucleocapsid.
- The nucleocapsid is sometimes surrounded a membrane called an envelope. Enveloped viruses are usually spherical because the envelope, unlike the capsid, is loose-fitting. Envelopes are lipid bilayers that contain proteins. Some proteins incorporate carbohydrates and are thus called glycoproteins. Glycoproteins usually protrude out of the envelope as spikes called peplomers. The function of peplomers is to form points of attachments to host cell receptors for entry into cells.
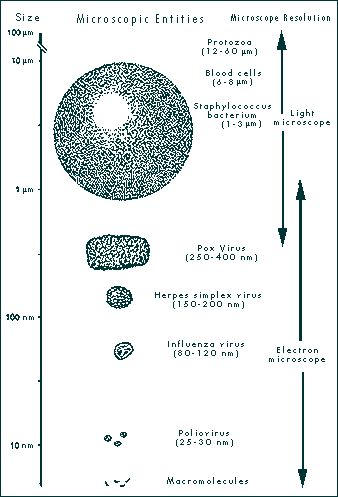 |
| FIGURE 1-1 SIZE OF MICROSCOPIC ENTITIES AND MICROSCOPE RESOLUTION. Viruses are smaller than the smallest bacteria and larger than macromolecules. They can be seen with the electron microscope. (Illustration demonstrates relative sizes and is not drawn to scale.)
From: Leland DS 1996 Clinical Virology, Saunders, New York |
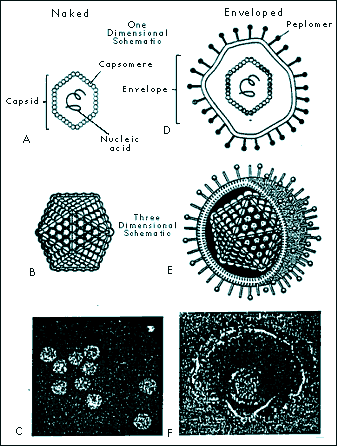 |
|
FIGURE 1-2 ICOSAHEDRAL CAPSID CONFIGURATION IN NAKED AND ENVELOPED VIRUSES. Naked icosahedral viruses appear cubic or crystalline (A, B, and C). Enveloped icosahedral viruses appear nearly spherical (D, E, F). (Fig. 1-2C from Ryan KJ (Ed), Sherris Medical Microbiology: An Introduction to Infectious Diseases, 3rd ed. Norwalk, CT: Appleton & Lange, 1994. Fig. 1-2F
From: Murray PR, Kobayashi GS, Pfaller MA, Rosenthal KS. Medical Microbiology, 2nd ed. St. Louis: Mosby Year Book, 1994, p 573 |
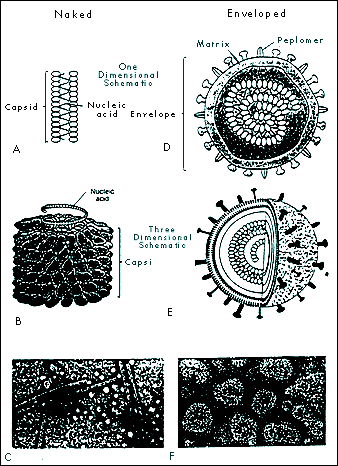 |
|
FIGURE 1-3 HELICAL CAPSID CONFIGURATION IN NAKED AND ENVELOPED VIRUSES. Naked helical viruses are cylindrical or rod shaped (A, B, and C). Enveloped helical viruses appear nearly spherical because the nucleocapsid (capsid and nucleic acid) may curl inside the envelope (D, E, and F). Fig. 1-3C from: Tortora et al. Microbiology, 4th ed., Benjamin Cummings, Redwood City CA, 1992, p 336. Fig. 1-31F
From: Ryan KJ (Ed), Sherris Medical Microbiology: An Introduction to Infectious Diseases, 3rd ed. , Appleton & Lange, Norwalk CT, 1994. |
The envelope is sometimes connected to the nucleocapsid by a matrix made of proteins (matrix proteins).
- Viruses that do not have an envelope are called "naked viruses".
- The term virion describes the mature viral particle capable of infecting other cells. In non-enveloped viruses, the virion consists of the nucleocapsid alone. In enveloped viruses, the nucleocapsid and the envelope make up the virion.
Enveloped viruses are usually comfortable in bodily fluids and are transmitted by such routes as blood transfusion, or mucosa to fluid contact, as in sexual contact.
Naked viruses, on the other hand, are usually transmitted via the intestinal route.
Major Enveloped Viruses Include the Following Families:
Hepadnaviridae (from Hep: liver; dna: DNA)| Hepatitis B (HBV). |
| HIV-1, Immunodeficiency syndrome |
| HIV-2, Immunodeficiency syndrome |
| Herpes simplex, types I and 2 |
| Varicella-zoster: Chicken pox and shingles |
| Cytomegalovirus (from cyto: cell; megalo: large) |
| Epstein-Barr causes infectious mononucleosis; implicated in chronic fatigue syndrome |
| Ebola virus (Ebola river, Zaire): Hemorrhagic fever |
| Marburg (Marburg, Germany): Hemorrhagic fever |
| Influenza A and B |
| Measles |
| Mumps |
| Parainfluenza |
| Respiratory syncytial virus: Flu-like syndrome |
Togaviridae (Toga: coat)
| Rubella - German measles |
| Eastern and Western equine encephalitis. Transmitted by mosquitoes. |
| Rabies |
Flaviviridae (Flavus: yellow)
| Hepatitis C |
Major Non-Enveloped (Naked) Viruses:
Adenoviridae (from adenoid tissues)| Adenovirus: Respiratory infections, gastroanteritis, cystitis |
| Polyomavirus, Papillomavirus (warts) |
| Poliovirus (polios: gray; myelitis: infection of the myelin in the spinal cord) |
| Echovirus (from E-enteric, C-cytopathogenic, H-human, 0-orphan): Aseptic meningitis |
| Coxsachie virus: Pharyngitis, aseptic meningitis, pericarditis, myocarditis |
| Hepatitis A: Infectious hepatitis |
| Rhinovirus (Rhino: nose): respiratory infection |
| Rotavirus (Rota: Wheel) - Severe diarrhea in infants and adults |
The Viral Life Cycle
Viruses have complex life cycles which demonstrate their extraordinary symbiosis with their hosts. They lack the tools for self sufficient growth and thus depend upon more advanced life systems for their existence.
Viral replication, in broad strokes, starts with viral attachment to host cells. Enveloped viruses use glycoprotein molecules on surface peplomers for binding to the cell membrane. Viral and cell membranes fuse and the viral nucleocapsid penetrates into the cell's cytoplasm. Naked viruses may be engulfed by the cell membrane in a process called endocytosis. Once inside the cell, the capsid is reconfigured to prepare it for replication.
In the replication phase, viral nucleic acid has the capacity to direct the host cell to manufacture all components of the mature virion. These are then assembled and mass produced. The virions are then released into the circulation in a process that may or may not involve cell destruction , or lysis.
A wave of viral particle carried through the blood stream to infect organs throughout the body is called a viremic episode. Many viruses travel free in the plasma. Others may attach themselves to platelets, lymphocytes or red blood cells.
Recent research has shown that the number of virions involved in infection is much more important than previously realized. In both HIV and hepatitis C, several billion new particles may be produced each day. The amount of viral concentration present at any one time is called the viral load. The immune system is placed under constant stress to deactivate these new infective particles, and to regenerate its own decimated cellular components.
Any reduction in the viral load offers an advantage to the immune system, and thus enhances the probability for easing clinical symptoms.
Commentaries on the Evolution of Viruses
Viruses are far from being static entities. As quintessential intracellular parasites they have developed, through millions of years of cohabitation with their hosts, astoundingly sophisticated structures, survival, and propagation mechanisms. They have adapted, modified their biological strategies, and incorporated genetic diversity and mutational capacity to cope with the changing ecology.
In the twentieth century, this ecology, namely the human reservoir, has changed dramatically. The eruptive world population and the mobility of the planet's inhabitants are two major factors responsible for the accelerated evolution of viruses into new frontiers of pathogenicity.
Most of the families of viruses mentioned above have produced new strains. Of great concern is the diversification of retroviruses and of Hepatitis B and C. Since 1985, for example, the following viruses are among the many that have been discovered: Human herpesvirus 6, 7, and 8; Hepatitis C; Hepatitis E; Morbillivirus, causing encephalitis; Hantaviruses causing the hantavirus pulmonary syndrome.
Principles of Viral Inactivation
The inactivation of viral particles in the test tube is possible by a number of interventions. The enveloped viruses are usually more sensitive to physico-chemical challenges than are naked virions. Chemical agents and other adverse conditions that affect the envelope invariably destroy the entire virus. These include drying, high temperature, freezing and thawing, pH below 6 or above 8, lipid solvents, hydrogen peroxide, chloroform, chemicals containing chlorine, ultraviolet irradiation, phenols, and ozone.
The test tube environment presents different parameters than, let us say, a biologically active substance e.g.., a serum, or a vaccine. In this case the virions in solution need to be destroyed while the components of the biological solution need to be preserved.
Ozone is an exemplary agent capable of deactivating a wide range of viruses while significantly sparing the biological integrity of their medium. There is, as concerns ozone, a therapeutic range of administration. Like many other drugs, ozone may be said to have a therapeutic window which defines its optimal levels of administration. Below the window, concentrations of administered ozone are poorly effective; within the window they function optimally; and above the window, deleterious effects take place.
Ozone offer distinct advantages over other antiviral agents. It is above all, a gas. When a gas is administered to a liquid, an immediate dispersion of the gas occurs, thus effecting reactions in the entire fluid volume. This may be contrasted to any fluid additive, such as hydrogen peroxide or detergents which mix with the fluid being treated much more slowly due to the physics of fluid-fluid dynamics. Furthermore, at the interface of the fluids, the concentrations of the chemicals are inordinately high and potentially toxic. As concerns the viability of cellular elements such as red and white blood cells, the oxidizing potential of ozone may be accurately calibrated so that it is fatal to virions, but innocuous to cells. Cells protect themselves from oxidative injury by means of protective enzymes (glutathione, superoxide dismutase, catalase), in contrast to virions which have no protection against oxidation.
The enveloped viruses are more fragile than the naked viruses. Their envelope, made up of lipids and glycoproteins are especially vulnerable to ozone's capacity for oxidation (oxidation is defined as the removal of electrons from compounds). Envelope lipids which are unsaturated, when exposed to ozone, become saturated, are cleaved at their double bond sites, and break apart. The envelope thus becomes torn and the viral capsid, by itself, cannot survive.
Although ozone's effects upon unsaturated lipids is one of its best documented biochemical actions, ozone is known to interact with proteins, carbohydrates, and nucleic acids. This becomes especially interesting when ozone inactivation of non-enveloped virions is considered.
The protective layer surrounding the DNA or RNA of virions (the capsid), is made up of proteins. Circulating freely in the bloodstream, the capsid's protein coat, in the case of naked viruses, is thus its first and last line of defense. Challenged by ambient ozone or its peroxides, the protein coating itself becomes denatured and incapable of sustaining its protective role (The viral nucleic acid material, by itself cannot survive). Indeed, when ozone comes in contact with capsid proteins, protein hydroxides and protein hydroperoxides are formed.
In the viral decontamination of a serum sample, it is assumed that several viral species may be present. Each virus has its own tolerance, and intolerance, for ozone challenge. There is an optimal concentration range, however, within which ozone may be administered to the serum sample, destroying its viral and bacterial occupants, and at the same time preserving the great majority of its biological activity (i.e., in the case of, vaccines, antigen purity; or in passive immunization, the sustenance of antibody titers).
The task of viral inactivation in vivo becomes more difficult. In this case we are dealing with a live patient and the awesomely complex dynamics determining the evolution of a viral infection. Some general guiding principles nevertheless stand out:
We have seen that viremic episodes represent invasions of virions into bodily fluids. In the case of acute infections such as Ebola, or more commonly in the flu syndrome, there may be one viremic episode, which in the first instance may be fatal, and in the second, may be hardly noticed by the patient. depending upon host factors.
In the case of chronic infections such as hepatitis or HIV, however, viremic episodes may occur numerous times in periods spanning several years. Viral load may be high, indicating a shift towards virus victory in relation to immune surveillance, defense and reserve, or may be low, indicating a quiescence within the viral life cycle.
Any intervention which will safely decrease the numbers of virions from the circulation will proffer an advantage to beleaguered immune function.
Ozone hemotherapy consists in the regular treatment of aliquots of blood with precise doses of ozone. The result is a culling action due to the direct action of ozone and the biologically active compounds it produces on viral particles, which translates into a progressive diminution in the viral load, and a corresponding enhancement of immune potency.
Another mechanism of viral inactivation with ozone is indirect. Subsequent to the treatment of blood with ozone, there exists in the serum a plethora of fragmented virions most of which are excreted through the kidneys. Some of these fragments, however, are processed by the immune system for the elaboration of its own defenses.
We know that each HIV-afflicted patient, for example, is infected with a unique subspecies of HIV virion. Both intact virions - and once destroyed, their fragments - have one of a kind antigenic structures (an antigen is defined as a substance capable of stimulating the production of antibodies). The immune system is thus able to manufacture organism-specific antibodies. The still poorly appreciated uniqueness of ozone therapy in this regard, is that - assuming the preservation of a minimum of immunocompetence - it provides the patient with an opportunity to make his own individualized autovaccine to the distinctive type of virus particle harbored.
Ozone: Clinical Methodology Ozone may be utilized for the therapy of a spectrum of clinical conditions. Routes of administration are varied and include external and internal (blood interfacing) methods. In the technique of ozone major autohemotherapy for viral diseases, an aliquot of blood is withdrawn from a virally-afflicted patient, anticoagulated, interfaced with an ozone/oxygen mixture, then re-infused. This process is repeated serially until viral load reduction is documented.
Ozone: A Review of Possible Mechanisms of Anti-viral Action
The viral culling effects of ozone in infected blood may recruit the following mechanisms:- Denaturation of virions through direct contact with ozone. Ozone, via this mechanism, disrupts viral envelope proteins, lipoproteins, lipids, and glycoproteins. The presence of numerous double bonds in these unsaturated molecules makes them vulnerable to the oxidizing effects of ozone which readily donates its oxygen atom and accepts electrons in these redox reactions. Double bonds are thus reconfigured, molecular architecture is disrupted and widespread breakage of the envelope ensues. Deprived of an envelope, virions cannot sustain nor replicate themselves.
- Ozone proper, and the peroxide compounds it creates, may directly alter structures on the viral envelope which are necessary for attachment to host cells. Peplomers, the viral glycoproteins protuberances which connect to host cell receptors are likely sites of ozone action. Alteration in peplomer integrity impairs attachment to host cellular membranes foiling viral attachment and penetration.
- Introduction of ozone into the serum portion of whole blood induces the formation of lipid and protein peroxides. While these peroxides are not toxic to the host in quantities produced by ozone therapy, they nevertheless possess oxidizing properties of their own which persist in the bloodstream for several hours. Peroxides created by ozone administration show long-term antiviral effects which serve to further reduce viral load. This factor may explain in part the reason for the fact that ozonated blood in the amount processed in usual treatment protocols is able to reduce viral load values in the total blood volume.
- Immunological effects of ozone have been documented. Cytokines are proteins manufactured by several different types of cells which regulate the functions of other cells. Mostly released by leucocytes, they are important in mobilizing the immune response. It has been found that ozone induces the release of cytokines which in turn activate a spectrum of immune cells. This is likely to constitute a significant avenue for the reduction of circulating virions.
- Ozone action on viral particles in infected blood yield several possible outcomes. One outcome is the modification of virions so that they remain structurally grossly intact yet sufficiently dysfunctional as to be nonpathogenic. This attenuation of viral particle functionality through slight modifications of the viral envelope, and possibly the viral genome itself, modifies pathogenicity and allows the host to increase the sophistication of its immune response. The creation of dysfunctional viruses by ozone offers unique therapeutic possibilities. In view of the fact that so many mutational variants exist in any one afflicted individual, the creation of an antigenic spectrum of crippled virions could provide for a unique host-specific stimulation of the immune system, thus designing what may be called a host-specific autovaccine.
Conclusion and Commentaries on Ozone Therapy and Research
In view of these considerations, it is evident that ozone presents fascinating opportunities for experimental and clinical research. Of all the antiviral agents known, none appear to offer the unique features of ozone, including its potent oxidizing power, and its gaseous nature. Indeed, as a gas, ozone is incomparably able to penetrate fluids with great ease and rapidity, delivering its biochemical actions almost instantaneously.
Research is needed to determine the effective range of ozone administration for the inactivation of each viral strain within all viral families. While this has been done for some members of major viral families, e.g., HIV and Hepatitis C, this knowledge applied to every main viral species will assist greatly in the precise methods for decontamination of mammalian products (serum, vaccines, etc.), and in the proper administration of therapeutic ozone in clinical situations. Research is needed, as well, to elucidate more precisely the mechanisms by which ozone decreases viral load.
Viral diseases are expanding worldwide. This is not a science fiction scenario but a regrettable fact. Old diseases are not only finding increased population reservoirs, but are also developing novel mechanisms of infectivity. Of special concern are emergent viruses, products of mutational creativity, never before encountered by humans, which are potentially devastating to health and life. Ozone has the unparalleled promise to find a major place in the armamentarium against these new plagues.
Viral load reduction by means of ozone blood treatment alleviates immune system fatigue. Due to the excess energy contained within the ozone molecule, it is theoretically likely that ozone, unlike antiviral options available today, will show effectiveness across the entire genotype and subtype spectrum. Ozone embodies unique physico-chemical and biological properties which suggest an important role in the therapy of a variety of viral conditions, most likely those mediated by lipid enveloped viruses, either as a monotherapy, or as an adjunct to standard treatment regimens.
Bibliography
- Akey DH, Walton DE. Liquid phase study of ozone inactivation of Venezuelan equine encephalitis virus. Applied Environmental Microbiology 1985; 50: 882
- Bocci V. Autohemotherapy after treatment of blood with ozone. A reappraisal. Journal of International Medical Research 1994 May-Jun; 22(3): 131-144
- Bocci V. Ozonation of blood for the therapy of viral diseases and immunodeficiencies. A hypothesis. Medical Hypotheses 1992 Sept; 39(1): 30-34
- Bocci V, et al. Studies on the biological effects of ozone: Generation of reactive oxygen species (ROS) after exposure of human blood to ozone. Journal of Biological Regulators and Homeostatic Agents 1998 July-Sept; 12(3): 67-75
- Bolton DC, Zee YC, Osebold JW. The biological effects of ozone on representative members of five groups of animal viruses. Environmental Research 1982; 27: 476-484
- Bolton DC, Tarkington BK, Zee YC, Osebold JW. An in vitro system for studying the effects of ozone on mammalian cell cultures and viruses. Environmental Research 1982; 27: 466-475
- Buckley RD, Hackney JD, Clark K, Posin C. Ozone and human blood. Archives of Environmental Health 1975; 30: 40-43
- Burleson GR, Murray TM, Pollard M. Inactivation of viruses and bacteria by ozone with and without sonication. Applied Microbiology 1975; 29: 340
- Cann AJ. Principles of Molecular Virology. Academic Press, San Diego, 1997
- Carpendale MT, Freeberg JK. Ozone inactivates HIV at noncytotoxic concentrations. Antiviral Research 1991; 16: 281-292
- Cardile V, et al. Effects of ozone on some biological activities of cells in vitro. Cell Biology and Toxicology 1995 Feb; 11(1): 11-21
- Evans AS, Kaslow RA (Eds) Viral Infections in Humans: Epidemiology and Control, Fourth Edition, Plenum, New York, 1997
- Garber GE, Cameron DW, Hawley-Foss N, et al. The use of ozone-treated blood in the therapy of HIV infection and immune disease - a pilot study of safety and efficacy. AIDS 1991; 5: 981-984
- Gonzalez-Peralta RP, Qian K, She JY, et al. Clinical implications of viral quasispecies heterogeneity in chronic hepatitis C. J Med Virology 1996; 49: 242-24
- Grob PJ. Hepatitis B: virus, pathogenesis, and treatment. Vaccine 1998; 16 Suppl S:11-16 Harrison TJ, Zuckerman AJ (Eds) The Molecular Medicine of Viral Hepatitis. Molecular Medical Science Series. John Wiley & Sons, New York, 1997
- Kim CK, Gentile DM, Sproul OJ. Mechanism of ozone inactivation of bacteriophage f2. Applied Environmental Microbiology 1980; 39: 210-218
- Konrad H. Ozone therapy for viral diseases. In: Proceedings 10th Ozone World Congress 19-21 Mar 1991, Monaco. Zurich: International Ozone Association 1991: 75-83
- Leland DS. Clinical virology. Saunders, Philadelphia, 1996
- Liang TJ, Hoofnagle JH (Eds) Hepatitis C. Academic Press, San Diego, 2000
- Paulesu L, Luzzi L, Bocci V. Studies on the biological effects of ozone: Induction of tumor necrosis factor (TNF-alpha) on human leucocytes. Lymphokine Cytokine Research 1991; 5: 409-412
- Razumovskii SD, Zaikov GE. Ozone and its reactions with organic compounds. Elsevier, Amsterdam , 1984
- Roy D, Wong PK, Engelbrecht RS, Chian ES. Mechanism of enteroviral inactivation by ozone. Applied Environmental Microbiology 1981; 41: 718-723
- Sunnen GV. Ozone in medicine: Overview and future directions. Journal of Advancement in Medicine 1988; 1(3): 159-174
- Turner BG, et al. Structural biology of HIV. Journal of Molecular Biology 1999 Jan; 285(1): 1-32
- Valentine GS, Foote CS, Greenberg A, Liebman JF (Eds). Active Oxygen in Biochemistry. Blackie Academic and Professional, London, 1995
- Vaughn JM, Chen Y, Linburg K, Morales D. Inactivation of human and simian rotaviruses by ozone. Applied Environmental Microbiology 1987; 48: 2218-2221
- Viebahn R. The Use of Ozone in Medicine. Haug, Heidelberg, 1994
- Wells KH, Latino J, Gavalchin J, Poiesz BJ. Inactivation of human immunodeficiency virus Type I by ozone in vitro. Blood 1991; 78(7): 1182-1890
- Yu BP. Cellular defenses against damage from reactive oxygen species. Physiological Reviews 1994 Jan; 74(1): 139-162